By Jared P. Vasiliauskas and Michael Viverito, of Power & Cronin, Ltd.
Each year, the World Anti-Doping Agency (“WADA”) releases its list of prohibited substances and methods, with an effective date of January 1 the following year. The 2022 List of Prohibited Substances and Methods (the “List”) was recently approved and released by WADA’s Executive Committee[1]. For a substance or method to be added to the list, it must first be determined that the substance meets at least two of three of the following: (1) the substance has the potential to enhance or enhances sport performance; (2) the substance represents an actual or potential health risk to the athlete; or (3) the substance violates the spirit of sport[2]. Below is an analysis of the key changes to the List beginning January 1, 2022. As with any prohibited substances, Athletes may apply for a Therapeutic Use Exemption for use of a prohibited substance; provided the Athlete meets the criteria outlined in the International Standards for Therapeutic Use Exemptions (“ISTUE”)[3].
S0: Non-Approved Substances
WADA has, for the first time, explicitly included BPC-157 as an example of a non-approved substance[4]. A non-approved substance is:
Any pharmacological substance which is not addressed by any of the subsequent sections of the List and with no current approval by any governmental regulatory health authority for human therapeutic use (e.g. drugs under pre-clinical or clinical development or discontinued, designer drugs, substances approved only for veterinary use) is prohibited at all times[5].
BPC-157 is an experimental peptide sold as a supplement which is used to help alleviate joint pain and injuries, and improve joint mobility by increasing vascular flow to the tendons and ligaments in order to increase angiogenic repair[6]. Common uses of BPC-157 include quickly healing body injuries, warding-off fatigue of the central nervous system as a result of time-intensive exercise, and retaining an anabolic state post-workout[7]. BPC-157 may be taken orally or via injection, with injection being the preferred medium when using the substance to address inflammation, sprains, ligament damage and pain[8]. As with all other Non-Approved Substances, BCP-157 is classified as a Specified Substance, and is prohibited at all times, both In- and Out-of-Competition[9].
S1.1: Anabolic Androgenic Steroids (AAS)
Tibolone has been transferred from S1.2 to S1.1 AAS[10]. The reclassification was based on the clinical effects of Tibolone as a synthetic oral androgen and the effects the substance has on the androgen receptor[11]. This is largely due to Tibolone’s conversion to the delta-4 tibolone metabolite[12].
S1.2: Other Anabolic Agents
Osilodrostat, a CYP11B1 inhibitor, is a new addition to List this year, with the substance being classified under S1.2. Historically, Osilodrostat is an oral supplement that has been used in treating Cushing’s Disease, which is caused by high exposure to cortisol levels over time[13]. However, Osilodrostat bears an unintended consequence of increasing testosterone circulation in users, leading to WADA’s inclusion of the substance on the List beginning in 2022[14].
All Anabolic Agents under S1 are classified as non-Specified Substances under the World Anti-Doping Code (the “Code”), and are prohibited at all times, both In- and Out-of-Competition[15].
S2.2: Peptide Hormones and Their Releasing Factors
S2.2 was revised for this edition of the List to split Section 2.2.3 and add Section 2.2.4. This is due to the addition of lonapegsomatropin, somapacitan, and somatrogon as examples of growth hormone analogues[16]. The key characteristics of the added examples are:
- Lonapegsomatropin, perhaps more well-known under the brand name “Skytrofa,” is injected into the body daily and is intended to be used as treatment for growth hormone deficiency by stimulating skeletal growth in users[17]. Skytrofa was approved by the Food and Drug Administration (FDA) in August 2021 for medical use in the United States[18].
- Sold under the brand name Sogroya, somapacitan is a long-acting human growth hormone derivative intended to be used as treatment for growth hormone deficiency[19]. While still administered as an injection, somapacitan is a once-weekly injection designed to reduce the frequency of the nuisance of multiple injections during the week for the user[20]. Somapacitan was approved by the FDA in August 2020 for medical use in the United States[21].
- Somatrogon is a long-acting human growth hormone molecule that is intended for the treatment of growth hormone deficiency and designed to be taken once-weekly[22]. As of the date of this article, Somatrogon has not been approved by the FDA for medical use in the United States[23].
The new Section 2.2.4 prohibits growth hormone releasing factors[24]. As with all Peptide Hormones and other prohibited substances under S2, all substances in S2.2.3 and S2.2.4 are classified as non-Specified Substances under the Code, and prohibited at all times, both In- and Out-of-Competition[25].
S3: Beta-2 Agonists
While the total daily dose remains 1600 micrograms over a 24-hour period, the daily dosing time intervals for salbutamol were modified from 800 micrograms over 12 hours to 600 micrograms over 8 hours starting from the time any dose is taken[26]. The intent behind this modification is to reduce the risk of any potential adverse analytical finding arising after high doses are taken at once[27]. As an example, an Athlete may take the maximum dose of 600 micrograms in the first 8 hours, a second maximum dose of 600 micrograms in the during the middle 8 hours, and the remaining 400 micrograms during the final 8 hours[28].
Taking inhaled salbutamol within the outlined intervals is an exception to the prohibitions of S3 of the List[29]. Should an Athlete seek doses in excess of 1600 micrograms over a 24-hour period, a Therapeutic Use Exemption would be necessary[30]. With limited exceptions, Beta-2 Agonists are prohibited at all times, and are classified as Specified Substances under the Code[31].
S6: Stimulants
While previous years have noted imidazole derivatives as an exception to prohibited In-Competition stimulants, WADA modified this exception to imidazoline derivatives, to distinguish between generic imidazole derivatives and sympathomimetic imidazolines[32]. Sympathomimetics are substances that mimic or modify the actions of endogenous catecholamines of the sympathetic nervous system[33]. Direct agonists directly activate adrenergic receptors while indirect agonists enhance the actions of endogenous catecholamines[34]. Sympathomimetics stimulate alpha-1 adrenergic receptors, beta-adrenergic receptors, and dopamine (D) receptors in various target tissues.
Cathine remains a prohibited stimulant. However, the footnote that accompanies the substance was modified to clarify that the urinary threshold of 5 micrograms per milliliter of Cathine refers to both isomers of norpseudoephedrine, i.e., the d-and the l-isomer[35].
Ethylphenidate, methylnaphthidate ((±)-methyl-2-(naphthalen-2-yl)-2- (piperidin-2-yl)acetate) and 4-fluoromethylphenidate have all been added to the List as substances banned while In-Competition due their composition making them close analogs to methylphenidate[36]. Methylphenidate is a central nervous stimulant used principally for treatment of attention deficit hyperactivity disorder (ADHD) by increasing the user’s ability to focus[37].
Hydrafinil (fluorenol) is a metabolite of modafinil that was added the List in S6.b as an example of modafinil and andrafinil analogue[38]. Hydrafinil can promote wakefulness and increasing mental alertness in users[39].
Under the Code, stimulants are only prohibited In-Competition, and the stimulants discussed above are classified under S6.b as Specified Stimulants[40]. Under the Code, “In-Competition” generally refers to “[t]he period commencing at 11:59 p.m. on the day before a Competition in which the Athlete is scheduled to participate through the end of such Competition and the Sample collection process related to such Competition.[41]”
S9: Glucocorticoids
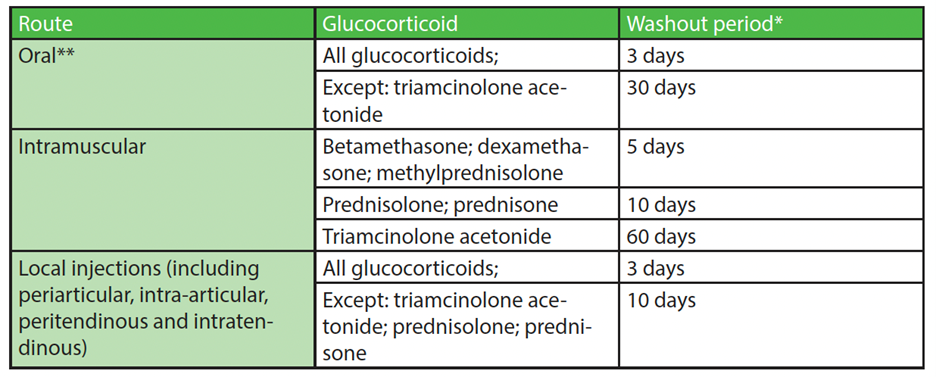
All injectable routes of administration of glucocorticoids are now prohibited during the In-Competition period[42]. Examples of injectable routes of administration include: intravenous, intramuscular, periarticular, intra-articular, peritendinous, intratendinous, epidural, intrathecal, intrabursal, intralesional (e.g., intrakeloid), intradermal, oromucosal, buccal, gingival sublingual, and subcutaneous[43]. Glucocorticoids may still be administered via inhaled or topical routes, including dental-intracanal, dermal, intranasal, ophthalmological and perianal, and are not prohibited In-Competition when used within the manufacturer’s approved doses or licensed therapeutic doses[44].
Athletes are urged to follow the minimum washout periods, as provided by WADA in the chart above, to reduce the risk of an adverse analytical finding[45]. The washout period refers to the time from the last administered dose to the start of the In-Competition period[46].
Please note that there may be a different time approved by WADA for a given sport.
The use of glucocorticoids is prohibited In-Competition only, and is considered a Specified Substance under the Code[47]. Athletes desiring to use a glucocorticoid by injection In-Competition are required to obtain a Therapeutic Use Exemption[48]. Alternatively, Athletes should consult their physician regarding the use of an alternative permitted medication.
Athletes should advise any physician administering local injections of glucocorticoids that periarticular or intra-articular injections may inadvertently result in intramuscular administration (prohibited In-Competition). If intramuscular administration is suspected, the washout periods for the intramuscular route should be observed[49].
In the instance of using a Prohibited Substance that is prohibited In-Competition only, Athletes may apply for a retroactive TUE if the Athlete used the substance Outside of Competition[50]. In preparing a case for a retroactive TUE, the Athlete should have a complete medical file of their use of the substance Prohibited In-Competition, and be prepared to submit their file with a completed Application for Therapeutic Use Exemption.
Jared P. Vasiliauskas is an attorney at Power & Cronin, a firm with experience in handling matters under the WADA Code, and International Standards for TUE on behalf of athletes. Those with questions can reach out to jared.vasiliauskas@powercronin.com and michael.viverito@powercronin.com at Power & Cronin, Ltd.
[1] https://www.wada-ama.org/en/media/news/2021-09/wada-publishes-2022-prohibited-list.
[2] Id.
[3] https://www.wada-ama.org/en/resources/therapeutic-use-exemption-tue/international-standard-for-therapeutic-use-exemptions-istue
[4] https://www.wada-ama.org/sites/default/files/resources/files/2022list_final_en.pdf
[5] Prohibited List, World Anti-Doping Agency S0 – Non-Approved Substances, effective January 1, 2022.
[6] https://www.wada-ama.org/en/media/news/2021-09/wada-publishes-2022-prohibited-list; https://limitlessmale.com/treatments/bpc-157/
[7] https://www.frontlinealternative.com/what-you-need-to-know-about-bpc-157
[8] https://vitality-sciences.com/peptides/what-is-bpc-157/
[9] Prohibited List, World Anti-Doping Agency S0 – Non-Approved Substances, effective January 1, 2022.
[10] https://www.wada-ama.org/sites/default/files/resources/files/2022list_explanatory_note_final_en.pdf
[11] Id.
[12] https://www.wada-ama.org/en/media/news/2021-09/wada-publishes-2022-prohibited-list
[13] https://www.mayoclinic.org/diseases-conditions/cushing-syndrome/symptoms-causes/syc-20351310
[14] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4799251/
[15] Prohibited List, World Anti-Doping Agency S1 – Anabolic Agents, effective January 1, 2022.
[16] Prohibited List, World Anti-Doping Agency S2 – Peptide Hormones, Growth Factors, Related Substances and Mimetics, effective January 1, 2022.
[17] https://reference.medscape.com/drug/skytrofa-lonapegsomatropin-4000162#0
[18] https://investors.ascendispharma.com/news-releases/news-release-details/ascendis-pharma-announces-us-food-and-drug-administration.
[19] https://academic.oup.com/jcem/article/105/4/e1847/5699635
[20] https://reference.medscape.com/drug/somapacitan-sogroya-4000054#10
[21] https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-weekly-therapy-adult-growth-hormone-deficiency
[22] https://www.opko.com/what-we-do/our-research/somatrogon
[23] Id.
[24] Prohibited List, World Anti-Doping Agency S2.2.4, effective January 1, 2022.
[25] Id.
[26] https://www.wada-ama.org/sites/default/files/resources/files/2022list_explanatory_note_final_en.pdf
[27] Id.
[28] Id.
[29] Prohibited List, World Anti-Doping Agency S3 – Beta-2 Agonists, effective January 1, 2022.
[30] https://www.wada-ama.org/sites/default/files/resources/files/2022list_explanatory_note_final_en.pdf
[31] Prohibited List, World Anti-Doping Agency S3 – Beta-2 Agonists, effective January 1, 2022.
[32] https://www.wada-ama.org/sites/default/files/resources/files/2022list_explanatory_note_final_en.pdf
[33] https://www.amboss.com/us/knowledge/Sympathomimetic_drugs/
[34] Id.
[35] https://www.wada-ama.org/sites/default/files/resources/files/2022list_explanatory_note_final_en.pdf
[36] https://www.frontiersin.org/articles/10.3389/fnins.2019.00124/full
[37] https://www.mayoclinic.org/drugs-supplements/methylphenidate-oral-route/description/drg-20068297; https://www.webmd.com/drugs/2/drug-12114-1516/methylphenidate-hcl-oral/methylphenidate-extended-release-suspension-oral/details
[38] Id.
[39] https://rhtp.org/hydrafinil/
[40] Prohibited List, World Anti-Doping Agency S6 – Stimulants, effective January 1, 2022.
[41] World Anti-Doping Code, 2021 at Appendix 1.
[42] https://www.wada-ama.org/en/media/news/2021-09/wada-publishes-2022-prohibited-list
[43] Id.
[44] Id.
[45] Id.
[46] Id.
[47] Prohibited List, World Anti-Doping Agency S9 – Glucocorticoids, effective January 1, 2022.
[48] https://www.wada-ama.org/sites/default/files/resources/files/2022list_explanatory_note_final_en.pdf
[49] Id.
[50] International Standards for Therapeutic Use Exemption, Article 4.1e.